For many, the beautiful and incomprehensible word “anodizing” is associated with complex physical and chemical technologies, laboratory conditions, and other scientific attributes. Few people know that this useful and uncomplicated process can be carried out using improvised means: to make anodizing of titanium and other metals realistically even at home. But what is it, and why is it needed for metal?
- What is anodized metal surface
- The advantages of anodized metal
- Different ways
- Warm method
- Cold method
- Anodic oxidation technology
- Types of Electrolytes
- Dangerous moments

What is anodized metal surface
The name anodizing is the process that occurs when using electrolyte and electric current of various sizes and allows you to get a strong oxide foam on the product, which increases the strength of steel and provides protection against corrosion. Strength and mechanical characteristics vary depending on the composition of the metal, the density and type of electrolyte, the magnitude of the anodic and cathodic effects, calculated according to special equations.
The protective coating itself is not applied, but is formed from the iron itself in the process of an electrochemical reaction. The technology used at home looks like this:
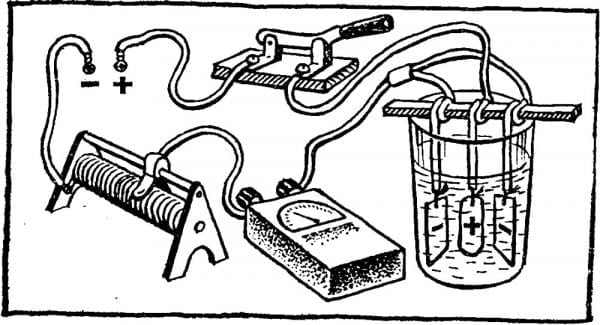
- An electrolyte is poured into a dielectric (non-conducting current) capacitance.
- A power supply unit is taken that can provide the necessary DC voltage at the output (it can be a battery or several batteries connected to electronic circuits).
- A clamp “+” is connected to the workpiece, and the object is immersed in a container with a solution.
- The “-” clamp is mounted on a plate of lead or stainless steel and is also lowered into the liquid.
- An electric current of the required value is connected, according to the electrochemical equation. Thanks to it, oxygen begins to be released on the surface of the product, contributing to the formation of a strong protective film.
to contents ↑
The advantages of anodized metal
Anodic oxidation (anodization) of various metals, carried out at home, of course, is much inferior to that which is carried out using industrial equipment. But, nevertheless, it is able to provide the product with a number of advantages:
- Increase corrosion resistance - due to the fact that the oxide film prevents the penetration of moisture to the metal base, providing reliable protection. The use of such a process on quickly rusting household items or discs and parts of household appliances can significantly extend their service life.
- Increase the strength of metal and steel: the oxidized coating is much more resistant to mechanical and chemical damage.
- Dishes processed in this way are non-toxic, resistant to prolonged heating, food does not burn on it.
- After anodized processing, metal products acquire dielectric properties (completely or almost not conduct current).
- The ability to conduct galvanic spraying of another metal (chrome, titanium). Done by hand, it is able to significantly increase the mechanical strength characteristics or increase decorative qualities (gold plating).
In addition, the process allows decoration.You can make color anodic oxidation. This result can be obtained by changing the equations of the supplied current strength and the density of the electrolyte (this is possible when anodizing of titanium and other solid materials is carried out) or using paint (more often for aluminum and other soft metals, but this process is also applied on solid bases). Objects painted in this way have a more even and deeper color.
The industrial method provides higher coating strength, the ability to conduct deep anodizing with the simultaneous application of cathodic electrochemical foam, which provides additional protection against corrosion. But even the anode-cathode treatment carried out at home will help make the disks or other parts of the moving mechanisms more durable, wear-resistant.
to contents ↑Different ways
There are two ways to carry out the process of oxidized steel treatment at home. Each of them has its advantages and disadvantages..
Warm method
The easiest process to do it yourself. It successfully proceeds at room temperature, using organic paint, allows you to create amazingly beautiful things. For this purpose, you can use both finished paints and pharmacy dyes (zelenka, iodine, manganese).
It will not be possible to obtain solid anodizing using this technology, the oxide foam is unstable, gives poor protection against corrosion, and is easily damaged. But, if the surface is painted after such a technique, then the adhesion of the coating to the base will be very high, nitro enamels or other paints will hold firmly, will not peel off, and will provide a high degree of corrosion protection.
to contents ↑Cold method
This technique, when carried out at home, requires careful control of the temperature, allowing it to fluctuate from –10 to + 10 ° C (the optimum temperature for conducting an electrochemical reaction according to the equation is 0 ° C). It is under such temperature conditions that the anodic and cathodic surface treatment proceeds most fully, slowly creating a strong protective oxide film. This allows the home craftsman to do hard anodizing with his own hands, providing steel with maximum protection against corrosion.
Using this technique, galvanic spraying can be done by applying copper, chrome or gold to the product, calculating the current strength using special equations. After such processing, it is very difficult to damage a steel part or discs. Corrosion protection is effective for many years, even when in contact with sea water, can be used to extend the life of underwater equipment.
to contents ↑A small minus is that the paint does not hold on such a surface. To impart color to the metal, the spraying method is used (copper, gold) or the electrochemical color change under the influence of electric current (current strength and electrolyte density are calculated according to a special equation).
Anodic oxidation technology
The whole process carried out by yourself can be divided into stages:
- The surfaces of disks and other metal parts are well cleaned of dirt, washed, and ground.
- Degreasing is carried out with white spirit or acetone.
- The required time is maintained in an alkaline solution (it is calculated according to the equation based on the structure of the material).
- After that, disks or other metal products are immersed in an electrolyte, where an anodic and cathodic reaction of an oxide film build-up is conducted.
- If cold processing of the product was carried out, then after removing it from the container, it should be thoroughly washed with acid, dried. Upon completion of this process, it is provided with a long reliable protection against corrosion.
- During the thermal process, the film will be porous, soft, requiring additional fixation, carried out by dipping in clean boiling water or by exposure to hot steam. Then it needs to be washed well.
Types of Electrolytes
At home, not only industrial chemical acid solutions are used, but also simple tools that can be found in any kitchen:
- By anodizing titanium, sodium chloride, sulfuric or phosphoric acid can be taken.
- For aluminum, oxalic, chromic or sulfuric acids are used.
- Instead of acids, table salt with baking soda can be used for anodic and cathodic processing of disks or other objects made of steel. You can make the necessary electrolyte by mixing 9 parts of concentrated soda solution with one part of saline.
The exposure time of disks, plates, other metal objects in an electrolyte tank under current is calculated according to the equation, based on physico-chemical parameters.
to contents ↑Dangerous moments
When using acids as an electrolyte, safety precautions must be strictly observed. Neglecting them can lead to accidents:
- Skin contact due to the use of a diluted preparation may cause minor burns. But such concentration is dangerous for the eyes, therefore protective glasses and gloves should not be neglected.
- Under the influence of current, oxygen and hydrogen vapors are released, which, when mixed, form an explosive gas. When working in a poorly ventilated area, you can get an explosion from any spark that could be fatal.
Observing safety precautions and stages of technological processing, you can get durable beautiful things: chrome car wheels, create jewelry “in gold”, add strength to details of household mechanisms, depending on the technologies used.











