Chemical metallization of plastics makes it possible to manufacture such industrial products and semi-finished products as light filters, printed circuit boards, catalysts, electroplating blanks and much more. Metallization can improve the resistance of plastics to mechanical stress, moisture and high temperature. In addition, parts that use a combination of plastic and metal weigh significantly less than metal.
- Technological features of metallization
- Features of the creation of galvanic coatings
- Adhesive properties of materials
- Vacuum metallization
- Metallization at home
- Copper plating
- Silver plating

Technological features of metallization
Copper is most often used as a sublayer surface for electroplating. The copper layer plays the role of a damper for plastic, due to which stresses are stabilized, which are inevitable with a significant difference in the thermal stress coefficients of such dissimilar materials.
The underlayer is additionally chromium-plated or nickel-plated as indicated in the figure below.
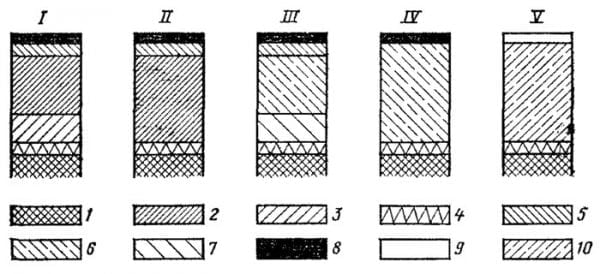
Explanations for the figure:
- Plastic.
- Gloss copper layer.
- Brushed copper layer.
- Chemical deposition metal.
- Gloss nickel layer.
- Semi-shiny nickel layer.
- Brushed nickel layer.
- Gloss chrome layer.
- Conversion layer.
- Brushed and shiny metallic layers.
The structural features of the compositions applied to the electrically conductive coating sublayer can vary significantly. We can talk about films of shiny, lightened, velor, blackened, patinated and other types. The task of films is not only to improve the appearance of products. For example, nickel-plated coatings extend the life of plastics. The fact is that nickel is capable of compressing plastic, significantly strengthening this material.
To create a galvanic coating, an electrolyte is needed. There are different types of electrolytes used, including:
- brilliant copper plating;
- electrolytes for nickel plating;
- special compositions on the basis of which velor-type coatings or coatings interspersed with solid particles are created.
Other metals, such as tin or zinc, are also used. However, before applying such metals, passivation is required, after which a film appears on the surface (with or without color). Such films protect the material from rust or plaque.
Chemical metallization of plastics is characterized by the fact that metal sublayers do not have high electrical conductivity. In any case, the conductivity is lower than in the case of electrolyte. Therefore, during electrochemical deposition, the density of the applied current should be negligible - from 0.5 to 1 Ampere per square decimeter. If the density is higher, a bipolar effect will occur, which will lead to dissolution of the coating near the place where there is contact with the conductive suspension.
In some cases, in order to avoid dissolution of the coating, copper or nickel is applied to the chemically deposited metal layer. And this is done at a low electric current density, but the subsequent layers are applied in the usual mode.
to contents ↑
Features of the creation of galvanic coatings
The galvanic layer, first of all, ensures the resistance of the metal to corrosion processes. During galvanization parts are in dense electrolytes. Thus, in order for the operation to be successful, special weighting agents are hung on the parts.
to contents ↑Electroplated coatings differ from metallic ones in that a much larger number of contacts will be needed to create them. The process of galvanizing plastics is also characterized by the complexity of the preparatory phase, since in this case it is more difficult to ensure good adhesion.
Adhesive properties of materials
Adhesion characterizes the quality of adhesion between elements of different types (in this case we are talking about adhesion between metal and plastic). The adhesion strength between metal and plastic coatings should be between 0.8 and 1.5 kilonewtons per meter — for peeling and equal to 14 megapascals — for breaking. The maximum possible adhesion achieved by modern technological means is approximately 14 kilonewtons per meter.
The adhesive qualities of materials are among the most complex phenomena. Suffice it to say that there is no unified theory that would fully answer all questions regarding the adhesion of dissimilar materials to each other.
From the point of view of chemical science, adhesion is a chemical relationship between different types of bodies. Chemical interactions can be seen on plastic surfaces. On such surfaces there are functionally active groups that come in contact with metals or coat metal surfaces with oxides.
The molecular approach interprets adhesion as a consequence of the presence of intermolecular forces on the interphase surface, the interaction of two poles, or the appearance of hydrogen bonds. This explains, for example, the adhesion of wet etched polyethylene films after drying.
From the point of view of electrical theory, adhesive qualities arise due to the fact that when a pair of bodies interact, a double electric layer appears. As a result, this layer does not allow bodies to move away from each other, since the electrostatic forces of mutual attraction of different charges work.
According to the diffuse theory (the most widely accepted), adhesion occurs due to intermolecular interactions, which are especially clearly manifested during the mutual penetration of molecules into the surface layers. At this time, a certain intermediate layer appears, as a result of which there is a lack of an obvious border between the materials.
And finally, the mechanical theory explains the adhesion by anchor adhesion of the protruding metal parts in the recesses on the plastic surface. Such depressions are very small in area (a few micrometers), however, when metal deposited by a chemical method gets into them, so-called mechanical locks appear.
Other parameters affect the adhesion, including the following:
- strength characteristics of plastic;
- the presence of favorable reactions of chemically active groups on a plastic surface;
- the presence of adhesion stimulants, which are otherwise called promoters (chromium and tin compounds, plasticizers);
- the absence of anti-promoters that hinder the strengthening or even destroy the intermediate layer;
- the structure of the chemically deposited metal, as well as the parameters at which this deposition occurs.
Vacuum metallization
The technology consists in spraying plastic with nichrome or aluminum using a vacuum. The application of metal to plastic using vacuum is carried out in a special chamber.The technique is widely used for applying a metal film to various surfaces, for example, car parts, plastic fittings, plumbing fixtures, lighting equipment, etc. To protect the metal, special paints and varnishes are used, characterized by increased hardness and resistance to moisture.

Metallization at home
Several techniques are known for self-applying metal onto a plastic coating. The most affordable of them is chemical. In this case, no special equipment is needed.
to contents ↑The metals used are silver and copper. The resulting film will be only a few microns in thickness, however, it will give the base a beautiful look with a metallic sheen.
Copper plating
Before processing, sand well and degrease the surface. If the part has bulges (defects), carefully reduce them to nothing. Pour abrasive onto the surface and wipe the surface with a swab. If we are dealing with polyacrylates, for degreasing, you will need a solution of caustic soda, in which the part is soaked for 24 hours. It is recommended to use gasoline for degreasing polyamides.
When the product is defatted, we wash it in distilled water, and then for a minute we keep it in a half-percent solution of tin chloride and hydrochloric acid (40 grams per liter). This process is called sensitization. Its purpose is to obtain a film of tin hydroxide on plastic.
After sensitization, we activate the surface. To do this, for 3-4 minutes, soak the part in a solution of silver nitrate (2 grams of silver per liter and 20 grams of ethyl alcohol per liter). Next, we place the product in a solution consisting of the following components:
- copper carbonate - 200 grams per liter;
- glycerin (90%) - 200 grams per liter;
- caustic soda (20%) - 1 liter;
The temperature of the solution should be 18-25 degrees. Processing time - 60 minutes.
to contents ↑Silver plating
We carry out preliminary processing of plastic in the same way as in the case of copper: sand and apply an abrasive. We wash the surface in soap and water, and then in distilled water.
Degrease the product with this solution:
- chromium anhydride - 100 grams per liter;
- iron sulfate - 10 grams per liter.
After degreasing, wash the part again in distilled water. We carry out sensitization in a solution of tin chloride (2 grams per liter). Next, we place the product in a solution that includes such components:
- silver nitrate - 3 grams per liter;
- caustic soda - 3.5 grams per liter;
- ammonia (25%) - 8 milliliters per liter;
- glucose - 2.5 grams per liter.
Recommended solution temperature is from 18 to 25 degrees. Processing time - 60 minutes. As a result, a uniform and shiny silver layer should appear. If somewhere there are heterogeneities, then this can be explained by insufficient surface degreasing. In this case, you need to remove the applied silver and repeat the work again.
To remove silver from plastic, you will need this solution:
- chromium anhydride - 10 grams per liter;
- sulfuric acid - 3 grams per liter.
It is recommended to process a uniform film with a varnish layer which will protect plastic. Further galvanic surface treatment is also possible.












Hello, is it possible or not, your way to use copper plating as a conductive layer? thanks
Hello, tell me, please, is it possible to apply your silvering method for PET or BOPP films?