Stainless steel is a high-quality alloyed metal with the addition of a number of chemicals that impart anti-corrosion properties. Due to alloying, steel becomes immune to moisture, air, and many aggressive environments. But sometimes even this material begins to deteriorate, ugly spots of rust appear on it. Why does stainless steel rust? There may be several reasons, and the main one is improper operation.
- Can a stainless steel rust?
- Factors determining the resistance of metal to corrosion
- Passive layer
- Types of stainless steel corrosion
- Crevice corrosion of stainless steels
- Total surface corrosion
- Pitting (pitting)
- Intercrystalline corrosion
- Contact corrosion
- The numerical equivalent of pitting resistance (PREN)
- Ways to protect stainless steel from the IWC
- Corrosion and surface treatment of stainless steel
- Stainless Steel Care

Can a stainless steel rust?
There are three groups of stainless steels, each of which has its own characteristics and specifics of application:
- Corrosion resistant steel. It has high resistance to corrosion in uncomplicated conditions - in the home, at work.
- Heat resistant steel. It has heat resistance, does not rust at elevated temperatures, and can be used in chemical plants.
- Heat resistant steel. Remains mechanically strong at high temperatures.
Thus, not all types of stainless steel are designed for operation in one or another aggressive environment. For example, the use of ordinary stainless steel in food production, frequent washing with chlorine-based products will cause rapid damage to the material. Similarly, the use of metal in sea water will lead to an increase in the corrosion rate by several times.
Also, rust often appears on a stainless steel after welding (heat treatment), which was carried out without observing certain rules. After mechanical damage to the metal, the consequences will be similar: pitting corrosion will occur at the defect site. Smooth, polished material usually rusts less intensely than rough: on the latter, corrosion elements can appear much faster.
Protection against rust is violated where incandescent scale has fallen, since alloying substances (mainly chromium) burn out due to a strong increase in temperature in non-heat-resistant steel. After burning holes, their edges and adjacent zones become susceptible to corrosion, although the deeper layers of the metal most often remain intact. To save the stainless steel will help processing etching pastes, special emulsions.
Other causes of stainless steel corrosion:
- contact of the material with ordinary carbon steel (including through tools that previously used to cut plain steel);
- regular brushing;
- ignoring the mechanical or chemical treatment of the weld.
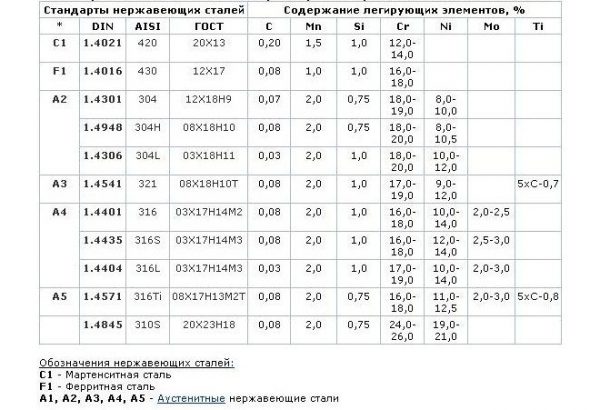
to contents ↑The cause of metal corrosion can be its initially low quality. The resistance of steel to rust is due to the presence of chromium in sufficient quantities. This element after exposure to water, air, acids and alkalis forms the thinnest impermeable layer that prevents the material from rusting. If there is little chromium in the composition or it is unevenly distributed, the creation and maintenance of the oxide layer becomes impossible.
Factors determining the resistance of metal to corrosion
So that the metal is not susceptible to corrosion, it must undergo passivation — the transition of the surface to an inactive (passive) state, in which a thin protective layer forms on it. A good stainless steel is quickly and easily passivated under normal atmospheric conditions - contact with oxygen from the air. The more chromium in the composition of the steel, the higher its passivation ability and anticorrosion properties.
In addition to chromium, alloying of steel is carried out using nickel. It also promotes passivation, but to a slightly lesser extent. Both metals give the highest corrosion resistance, although other elements can also be introduced into the composition of the steel: copper, niobium, molybdenum. To enhance the protective properties, any additives should be in a standard state, and when their structure is changed, corrosion resistance decreases (for example, when chromium passes into the form of nitride, carbide). This can happen during contact with strong acids: sulfuric, hydrochloric, hydrofluoric.
to contents ↑Passive layer
By a passive layer is meant a thin oxide film that forms on steel after the reaction of chromium with oxygen. It favorably affects only the properties of stainless steel: on ordinary steel, oxygen, when interacting with iron atoms, provokes the formation of small pores and the appearance of rust. The corrosion layer will also be called passive, because it is reactively inert with respect to the environment.
to contents ↑Types of stainless steel corrosion
According to the type of development, the reason for the appearance and signs, several types of stainless steel corrosion are distinguished.
Crevice corrosion of stainless steels
Crevice corrosion is a common form of stainless steel rusting. It develops where there is a small gap in the structure, for example, when water penetrates under the fasteners inside the product. The second surface in this case is usually a rubber seal, a gasket, and sometimes a metal element.
The mechanism of formation of crevice corrosion is as follows:
- The accumulation of aggressive ions in the gap, the displacement of oxygen.
- The appearance of the anode in the gap (the material outside the gap in this case plays the role of the cathode).
- Corrosion due to changes in acidity and electrochemical reactions.
to contents ↑To prevent crevice corrosion, it is necessary to properly design structures. It is important to provide cathodic protection, which will reduce acidity, as well as improve fluidity of the medium.
Total surface corrosion
General corrosion is the uniform violation of the metal structure in part of the surface layer. It causes the destruction of the oxide film on most of the product or over its entire area. Typically, the cause is contact with strong alkalis, acids, iodine, fluorine, and bromine compounds. The main "enemy" of the stainless steel is chlorine - that is why chlorine-containing detergents cannot be used to clean it.
to contents ↑Pitting (pitting)
Most pitting corrosion stainless steels, as well as alloys based on aluminum and nickel, are susceptible. Unlike ordinary steel, which often suffers from general surface corrosion, such materials in most cases are covered precisely by pitting - minor defects. Local destruction of the passive layer occurs in such situations:
- scratching, mechanical damage;
- local change in steel composition;
- point effect of chlorine ions, sulfur, halides;
- temperature rise.
Spot rusting is considered the most common among different types of stainless steel. Because of it, holes appear in the tanks, small cracks in the pipes, tanks. Usually their diameter is not more than 1 mm, while the depth can be significant - this is the insidiousness of this phenomenon.As in the case of crevice corrosion, the concrete pitting will act as the anode, and the rest (intact) surface will become the cathode. Adding molybdenum to stainless steel during its production increases the resistance of products to pitting corrosion.
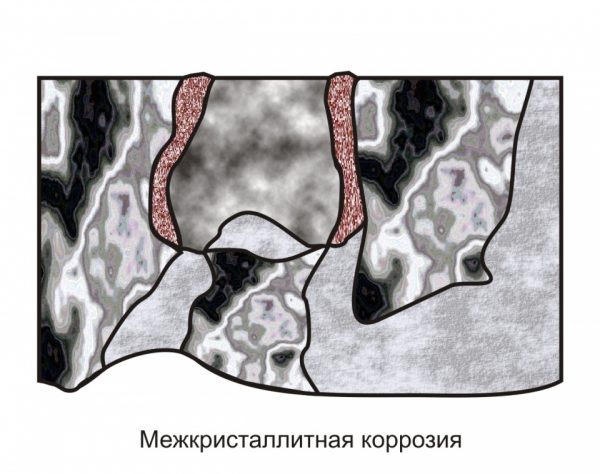
to contents ↑Intercrystalline corrosion
This process has another name - intergranular corrosion of stainless steels (MCC). It occurs with a sharp increase in temperature, which happens, for example, during welding. Rusting begins if, with the participation of heating along the grain boundaries, chromium carbamide appears, that is, the structure of this dopant changes dramatically. For ferritic steel, a sufficient temperature for the formation of foci of corrosion is +900 degrees, for austenitic steel - +450 degrees.
to contents ↑Contact corrosion
This type of corrosion develops when direct contact of dissimilar metals with each other under the influence of electrolytes. For example, this happens when different metal products are docked in an aggressive conductive medium - sea water. As a result, steel spoils locally, and less noble metals can even dissolve completely.
The numerical equivalent of pitting resistance (PREN)
RREN is a reference indicator, it shows the tendency of different types and brands of stainless steel to the appearance of pitting. The numerical equivalent of pitting resistance is used as a guideline, but not as an absolute guide to determining corrosion resistance.
Typically, molybdenum, chromium, and nitrogen are the most resistant to spot rusting as additives during alloying. The higher the RREN, the more resistant the steel will be to pitting. Here is the reference information for RREN:
| steel grade | Rren |
|---|---|
| 444 | 25 |
| 430 | 16 |
| 304 | 19 |
| 316 | 26 |
| 304LN | 21 |
| 904L | 36 |
| 316ln | 27,5 |
| SAF 2507 | 42 |
| Zeron 100 | 41 |
Ways to protect stainless steel from the IWC
It can sometimes be difficult to clean the surface from rust, especially with deep penetration of the defect. A number of methods against intergranular corrosion have been developed, here are the main ones:
- Annealing (stabilization). Ferritic steels are treated with high temperatures (+ 750 ... + 900 degrees), due to which the concentration of chromium on the surface increases, while the distribution of the element becomes more uniform.
- Carbon reduction. If the concentration of the substance is less than 0.03%, the metal will become practically not susceptible to intergranular corrosion.
- Quenching in water. This method is applicable to austenitic steel; it helps chromium carbides to transition into a more suitable form and concentrate on the grain boundaries of the metal.
to contents ↑To remove the tendency to MCC from the stainless steel, new additives are also introduced into it: titanium, tantalum, niobium, but this leads to a serious increase in the cost of the material. Their number should be 5-10 times greater than the carbon norm, and then the metal will not be susceptible to rust.
Corrosion and surface treatment of stainless steel
Corrosion can be removed chemically - use special rust converters. Also, the surface of stainless steel products can be processed by milling, grinding, grinding, polishing. The choice of a specific technique depends on the preferences of the specialist and a number of other conditions.
The selection of a method for the prophylactic treatment of metal will be determined by the initial corrosion resistance of a particular steel grade. On rough surfaces, pitting corrosion is more often formed, and on smooth rust spots rarely appear. Grades 304, 316 when used in seawater conditions quickly rust, they need to be protected more carefully.
to contents ↑Stainless Steel Care
In order for stainless steel items to remain attractive and functional for a long time, you need to take good care of them.Under normal conditions, products are regularly, at least every 6 months, washed with mild surfactants without chlorine and ammonia. In harsh climates, washing should be more frequent. When stains are identified, they are immediately thoroughly wiped, the pits are sealed with special means. Care will help extend the life of stainless steel products and reduce the risk of corrosion.












Good evening. And how to protect stainless steel in sea water in the pool.